| Identification | Back Directory | [Name]
(S)-(R)-JOSIPHOS | [CAS]
162291-02-3 | [Synonyms]
(S)-(R)-JOSIPHOS
JOSIPHOS SL-J001-2
97% (S)-(R)-JOSIPHOS
(S)1 (1R)2(DIPHENYLPHOSPHINO)FERROCENYL&
(S)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]
(S)1[(1R)2(diphenylphosphino)ferrocenyl] et-dicyclohexylphos
(S)-1-[(1R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]ETHYLDICYCLOHEXYLPHOSPHINE
(S)-1-[(RP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine
(S,S)-1-[1-(DICYCLOHEXYLPHOSPHINO)ETHYL]-2-(DIPHENYLPHOSPHINO)FERROCENE
(S)-(+)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]ETHYLDICYCLOHEXYLPHOSPHINE
(S)-(+)-1-[(R)-2-(Diphenylphospino)ferrocenyl] ethylbicyclohexylphosphine
(2S)-1-[(1S)-1-(Dicyclohexylphosphino)ethyl]-2-(diphenylphosphino)ferrocene
(S)-1-[(RP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine >=97%
Ferrocene, 1-[(1S)-1-(dicyclohexylphosphino)ethyl]-2-(diphenylphosphino)-, (2S)-
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenylethyl]dicyclohexylphosphine ethanol adduct
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ethanol adduct (S)-(R)-JOSIPHOS
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ethanol adduct,97% (S)-(R)-JOSIPHOS
(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphineethanoladduct,min.97%(S)-(R)-JOSIPHOS
(S)-(R)-Josiphos, Josiphos SL-J001-2, (2S)-1-[(1S)-1-(Dicyclohexylphosphino)ethyl]-2-(diphenylphosphino)ferrocene (acc to CAS) | [Molecular Formula]
C36H44FeP2 10* | [MDL Number]
MFCD00800285 | [MOL File]
162291-02-3.mol | [Molecular Weight]
594.53 |
| Questions And Answer | Back Directory | [Reactions]
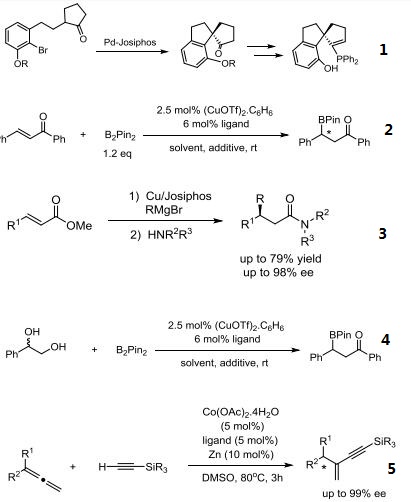
- Ferrocenylphosphine ligands of the type cpFecp(PR2)(*CH(CH3)PR'2) are a class of asymmetric ligands developed at Solvias in Basel, Switzerland. Ligands of this type are currently used industrially in the stereoselective synthesis of commercial products. A unique feature of these bidentate ligands is the presence of a fixed phosphine moiety and a stereogenic,functionalized side chain, which can be easily modified to accommodate electronic and steric requirements. Based on a versatile synthetic procedure starting with optically active ferrocenes of the type cpFecp(PR2)(*CH(CH3)X) [X = OAc or NR2], a variety of donor atoms can be introduced into the side chain.4 These ferrocene based phosphine ligands have wide application in the stereoselective hydrogenation of substituted acetamidoacrylates, enol acetates, β-ketoesters and simple alkenes.
- Pd-catalyzed, enantioselective, intramolecular α-substituted cyclic ketones: facile synthesis of functionalized chiral spirobicycles.
- Asymmetric boron conjugate addition of α,β-unsaturated carbonyl compounds catalyzed by
- CuOTf/Josiphos under non-alkaline conditions.
- Chiral amides via copper-catalyzed enantioselective conjugate addition.
- Ruthenium-catalyzed enantioselective synthesis of β-amino alcohols from 1,2-diols by “borrowing hydrogen”.
- Cobalt-catalyzed asymmetric addition of silylacetylenes to 1,1-disubstituted allenes.
|
|
|