| Identification | Back Directory | [Name]
t-BuBrettPhos Palladacycle Gen. 3 | [CAS]
1536473-72-9 | [Synonyms]
t-BuBrettPhos Palladacycle Gen. 3
Methanesulfonato 2-Di-t-butylphosphino-3,6-dimethoxy-2'-4' -6'-tri-i-propyl-1,1'-bipheny)(2'-amino-1,1'-biphenyl-2-yl)palladium(II)
Methanesulfonato(2-(di-t-butylphosphino)-3,6-dimethoxy-2',4',6'-tri-i-propyl-1,1'-biphenyl)(2'-amino-1,1'-biphenyl-2-yl)palladium(II), min. 98% | [Molecular Formula]
C44H62NO5PPdS | [MDL Number]
MFCD25976528 | [MOL File]
1536473-72-9.mol | [Molecular Weight]
855.433 |
| Questions And Answer | Back Directory | [Reaction]
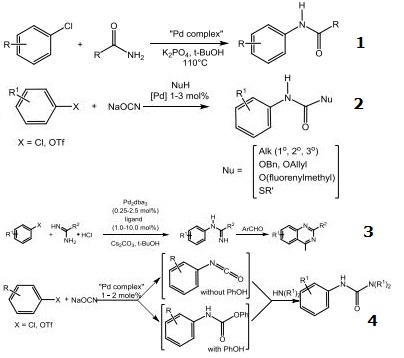
- Palladium catalyst used for the arylation of primary amides.
- Palladium catalyst used for the synthesis of N-aryl carbamates.
- Palladium catalyst used for the N-monoarylation of amidines.
- Palladium catalyst used for the cross-coupling of aryl chlorides and triflates with sodium cyanate – a practical synthesis of unsymmetrical ureas.
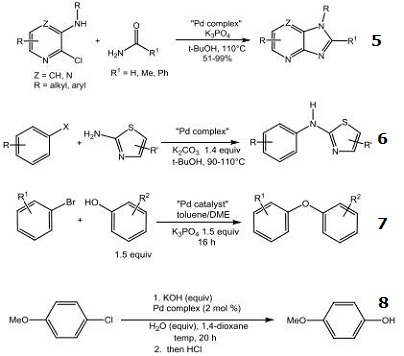
- Palladium catalyst used in the synthesis of imidazo[4,5-b]pyridines and imidazo[4,5]pyrazines through amidation of 2-chloro-3-amino-heterocycles.
- Palladium catalyst used in the N-arylation of 2-aminothiazoles
- Palladium catalyst used in the synthesis of diarylethers under mild conditions.
- Palladium catalyst used in the hydroxylation of aryl and heteroaryl halides.
|
| Hazard Information | Back Directory | [Uses]
tBuBrettPhos Pd G3 has been used as a precatalyst for the N-arylation of amino acid esters with aryl triflates under mild reaction conditions and minimal racemization of the amino acid ester. It may also be used to catalyze the conversion of aryl halides to phenols in the presence of benzaldoxime as a hydroxide surrogate. | [General Description]
tBuBrettPhos Pd G3 is a third generation (G3) Buchwald precatalyst that can be used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. It is air-, moisture-, and thermally-stable and is highly soluble in a wide range of common organic solvents. Some of its unique features include lower catalyst loadings, shorter reaction time, efficient formation of the active catalytic species and accurate control of ligand: palladium ratio. |
|
|