| Identification | Back Directory | [Name]
ETHYL 3-OXO-4-(TRIPHENYLPHOSPHORANYLIDENE)BUTYRATE | [CAS]
13148-05-5 | [Synonyms]
ETHYL 3-OXO-4-(TRIPHENYLPHOSPHORANYLIDENE)BUTYRATE
Ethyl 3-oxo-4-(triphenylphosphoranylidene)butanoate
Ethyl 3-oxo-4-(triphenylphosphoranylidene)butyrate,97%
[3-(ETHOXYCARBONYL)-2-OXOPROPYLIDENE]TRIPHENYLPHOSPHORANE
4-(Triphenylphosphoranylidene)acetoacetic Acid Ethyl Ester
3-Oxo-4-(triphenylphosphoranylidene)butanoic Acid Ethyl Ester
[3-(Ethoxycarbonyl)-2-oxopropylidene]triphenylphosphorane, 98 %
Ethyl 3-oxo-4-(triphenylphosphoranylidene)butyrate, [3-(Ethoxycarbonyl)-2-oxopropylidene]triphenylphosphorane | [Molecular Formula]
C24H23O3P | [MDL Number]
MFCD00192162 | [MOL File]
13148-05-5.mol | [Molecular Weight]
390.41 |
| Chemical Properties | Back Directory | [Appearance]
Off-White Powder | [Melting point ]
101-106 °C
| [Boiling point ]
544.0±52.0 °C(Predicted) | [density ]
1.18±0.1 g/cm3(Predicted) | [storage temp. ]
-20°C Freezer | [solubility ]
Acetone, Dichloromethane, Methanol | [form ]
solid | [color ]
Off-White to Pale Yellow | [Stability:]
Moisture Sensitive |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Powder | [Uses]
A useful synthetic intermediate in the production of antibiotics | [Description]
Ethyl 3-oxo-4-(triphenylphosphoranylidene)butyrate is a Wittig reagent for the stereoselective synthesis of (Z)-enones; annulation reagent for the synthesis of
cyclohexenones. | [reaction suitability]
reaction type: C-C Bond Formation | [Synthesis]
Ethyl 4- (Triphenylphosphoranylidene)acetoacetate is prepared in two steps from ethyl or
methyl 4-chloroacetoacetates (see Ethyl 4-Chloroacetoacetate), respectively, in 58-68% overall yield (eq 1).
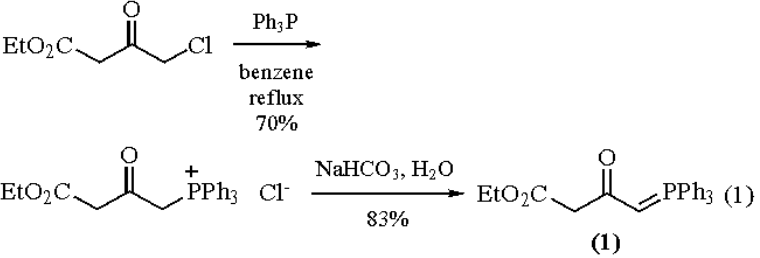 |
|
|