| Identification | Back Directory | [Name]
TAK-438 | [CAS]
1260141-27-2 | [Synonyms]
AK-438
CS-597
TAK-438
Vonaprazan
TAK 438; TAK438
TAK-438 USP/EP/BP
4-Aminopyrazolo[3
Vorolazan fumarate
Fumarate vonolasan
Vonorasen fumarate
Wonarazan Fumarate
TAK 438,Vonoprazan
vonaprazan(TK-438)
fuMarate vonoprazan
Vonaprazan(TAK-438)
vonoprazan(tak-438)
Vonoprazan FuMarate
Vonoprazon Fumarate
vomoprazan fumarate
Vonoprazan-025-Salt2
Vonolazan Fumarate API
Vonoprazan fumarate API
TAK-438 ISO 9001:2015 REACH
TAK-438
Vonoprazan fumarate
Vonoprazan fumarate(TAK-438)
vonoprazan(tak-438)1260141-27-2
TAK-438,1260141-27-2 Fluorine Prazan
VONOPRAZAN FUMARATE (TAK-438);TAK-438
Vonoprazan Vonoprazan onoprazan Vonoprazan fumarate
Vonoprazan Fumarate
DISCONTINUED PLEASE SEE V767013
1-[5-(2-fluorophenyl)-1-pyridin-3-ylsulfonylpyrrol-3-yl]-N-methylmethanamine
1-(5-(2-FLUOROPHENYL)-1-(PYRIDIN-4-YLSULFONYL)-1H-PYRROL-3-YL)-N-METHYLMETHANAMINE
5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine 2-butenedioate
1H-Pyrrole-3-methanamine, 5-(2-fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-, 2-butenedioate (1:1)
1-[5-(2-fluorophenyl)-1-pyridin-3-ylsulfonylpyrrol-3-yl]-N-methylmethanamine,(E)-4-oxopent-2-enoic acid
5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine 2-butenedioate (Vonoprazan)
Vonoprazan Fumarate-D3Q: What is
Vonoprazan Fumarate-D3 Q: What is the CAS Number of
Vonoprazan Fumarate-D3
5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine 2-butenedioate (Vonoprazan fumarate)
5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine 2-butenedioate TAK 438 | [EINECS(EC#)]
250-635-4 | [Molecular Formula]
C21H20FN3O6S | [MDL Number]
MFCD18633280 | [MOL File]
1260141-27-2.mol | [Molecular Weight]
461.463 |
| Chemical Properties | Back Directory | [storage temp. ]
Store at -20°C | [solubility ]
insoluble in H2O; insoluble in EtOH; ≥18.9 mg/mL in DMSO | [form ]
solid | [InChIKey]
ROGSHYHKHPCCJW-WLHGVMLRSA-N | [SMILES]
N1(S(C2=CC=CN=C2)(=O)=O)C(C2=CC=CC=C2F)=CC(CNC)=C1.C(O)(=O)/C=C/C(O)=O |
| Hazard Information | Back Directory | [Description]
Vonoprazan fumarate (Takecab®), discovered and developed by
Takeda and Otsuka, was approved by the PMDA of Japan in
December 2014, and is indicated for the treatment of gastric ulcer,
duodenal ulcer and reflux esophagitis. Vonoprazan fumarate has
a novel mechanism of action called potassium-competitive acid
blockers, which competitively inhibit the binding of potassium
ions to H+, K+-ATPase (also known as the proton pump) in the final
step of gastric acid secretion in gastric parietal cells. Vonoprazan
does not inhibit Na+, K+-ATPase activity even at concentrations 500
times higher than that of their IC50 values against gastric H+,
K+-ATPase activity. Furthermore, the drug is unaffected by the
gastric secretory state, unlike PPIs. | [Uses]
Vonoprazan Fumarate is a novel potassium-?competitive acid blocker for the treatment of acid-?related diseases. | [Application]
Vonoprazan fumarate is an oral, newly synthesised potassium-competitive acid blocker (P-CAB) with antisecretory activity. It is also a proton pump inhibitor (PPI) reversibly inhibiting H+/K+, ATPase. It is mainly used in the treatment of acid-related diseases such as GERD and peptic ulcer disease. | [Mechanism of action]
Vonoprazan fumarate has a novel mechanism of action called potassium-competitive acid blockers, which competitively inhibit the binding of potassium ions to H+, K+-ATPase (also known as the proton pump) in the final step of gastric acid secretion in gastric parietal cells. Vonoprazan does not inhibit Na+, K+-ATPase activity even at concentrations 500 times higher than that of their IC50 values against gastric H+, K+-ATPase activity. Furthermore, the drug is unaffected by the gastric secretory state, unlike PPIs. | [Synthesis]
Commercially
available 2-fluoroacetophenone (283) was brominated to yield
a-bromo-acetophenone derivative 284. This compound was
treated with ethyl 2-cyanoacetate (285) under basic conditions
to provide ketoester 286 in essentially quantitative yield. Next,
intramolecular condensation of 286 upon treatment of 4 M HCl
furnished the tri-substituted pyrrole 287 in 53% yield. Reduction
of the chloride under hydrogenolytic conditions facilitated arrival
at pyrrole 288, albeit in just 18% yield. Subsequent diisobutylaluminium
hydride (DIBAL) reduction, followed by the oxidation with
tetrapropylammonium perruthenate (TPAP) and 4-methylmorpholine
N-oxide (NMMO) afforded the corresponding aldehyde 290 in 60% yield across the 2 steps. Next, N-pyrrole substitution with pyridine-
3-sulfonyl chloride 291 gave rise to N-sulfonylpyrrole 292 in
82% yield. Reductive amination of 292 afforded amine 293, which
was treated with fumaric acid (294) via co-crystallization to provide
vonoprazan fumarate (XXXVI) in 74% for the two steps.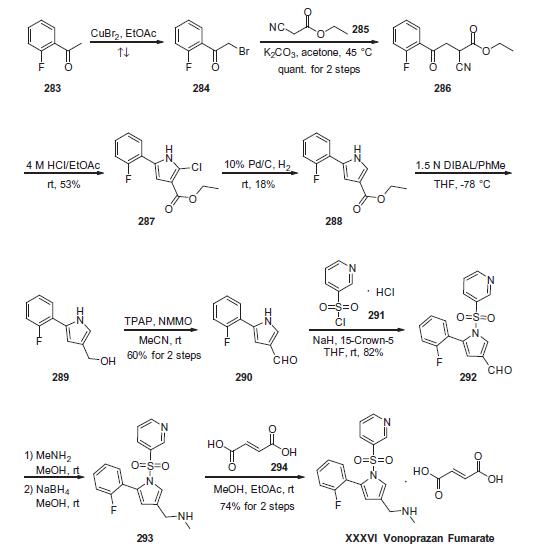
| [target]
H,K-ATPase | [storage]
Store at -20°C | [References]
[1]. yasunobu hori, jun matsukawa, toshiyuki takeuchi, et al. a study comparing the antisecretory effect of tak-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. journal of pharmacology and experimental therapeutics, 2011, 337:797-804.
[2]. jun matsukawa, yasunobu hori, haruyuki nishida, et al. a comparative study on the modes of action of tak-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. biochemical pharmacology, 2011, 81:1145-1151. |
|
|